NCERT Solutions for Class 9 Science Chapter 4 – CBSE Term II Free PDF Download
NCERT Solutions for Class 9 Science Chapter 4 Structure of The Atom at BYJU’S provides students with solutions to all the questions provided in the NCERT Class 9 textbook, which is on the lines of the CBSE board. This article provides students with the study source material that is crafted by a team of experts with in-depth knowledge of the subject. Solving NCERT Solutions for Class 9 Science would enable students to score maximum marks in the Class 9 CBSE Science Term II exam.
The language used while preparing the NCERT Solutions for Class 9 Science by our experts at BYJU’S is proficient and to the understanding of the Class 9 students, thereby assisting them in understanding the concept better. This is established on the concept-based approach. These NCERT Solutions are curated keeping in mind the answering method that is promoted by the CBSE and hence the allotment of marks in CBSE Term II examinations is taken into consideration for students to lay out their answers correspondingly and hence to score maximum marks. The NCERT textbook provided by the board is designed by the panel taking into consideration the latest in the field of science and technology.
Download PDF of NCERT Solutions for Class 9 Science Chapter 4 – Structure Of The Atom
Access Answers to NCERT Class 9 Science Chapter 4 – Structure Of The Atom (All in text and Exercise Questions solved)
Exercise-4.1 Page: 47
1. What are the canal rays?
Solution:
The radiations that are positively charged are canal rays. This discovery was crucial in the discovery
of another subatomic particle that was positively charged – proton.
2. If an atom contains one electron and one proton, will it carry any charge or not?
Solution:
Since a proton is a positively charged particle and an electron is a negatively charged particle, the net
charge becomes neutral as both the particles neutralizes each other.
Exercise-4.2 Page: 49
1. On the basis of Thompson’s model of an atom, explain how the atom is neutral as a whole.
Solution:
As per Thompson’s model of an atom,
(i) An atom contains a positively charged sphere in which the negatively charged electrons are implanted.
(ii) Electrons and protons are equal in magnitude, hence an atom on the whole is electrically neutral.
2. On the basis of Rutherford’s model of an atom, which subatomic particle is present in the nucleus of an atom?
Solution:
As per Rutherford’s model of an atom, the positively charged protons are the ones that are present in the atom.
3. Draw a sketch of Bohr’s model of an atom with three shells.
Solution:

4. What do you think would be the observation if the ∝– particle scattering experiment is carried out using a foil of a metal other than gold?
Solution:
In the ∝ – particle scattering experiment, when any other metal foil is used instead of gold, the observation would remain the same. This is because the structure of an atom when considered individually remains the same.
Exercise-4.2.4 Page: 49
1. Name the three subatomic particles of an atom.
Solution:
An atom consists of three subatomic particles:
- Protons – positively charged
- Electrons – negatively charged
- Neutrons – neutral in nature ( no charge )
2. Helium atom has an atomic mass of 4 u and two protons in its nucleus. How many neutrons does it have?
Solution:
Given: Atomic mass of helium atom = 4u, 2 protons in helium nucleus
Atomic mass = number of protons + number of neutrons
4 = 2 + number of neutrons
Number of neutrons = 4 – 2 = 2
Hence, Helium has 2 neutrons.
Exercise-4.3 Page: 50
1. Write the distribution of electrons in Carbon and Sodium atoms.
Solution:
Distribution of electrons in Carbon atoms:
The atomic number of Carbon is 6
Number of electrons is equal to the number of protons in carbon atom i.e., 6
The distribution of electrons in carbon atom is K – 2, L – 4
Distribution of electrons in sodium atoms:
The atomic number of Sodium is 11
Number of electrons is equal to the number of protons in sodium atom i.e., 11
The distribution of electrons in sodium atom is K – 2, L – 8, M – 1
2. If K and L shells of an atom are full, then what would be the total number of electrons in the atom?
Solution:
K shell can hold 2 electrons
L shell can hold 8 electrons
Hence, when both the shells are full, the total number of electrons present in the atom = 2+8 = 10 electrons.
Exercise-4.4 Page: 52
1. How will you find the valency of chlorine, sulphur and magnesium?
Solution:
The definite combining capacity of the atoms of each element, wherein electrons are lost, gained or shared to make the octet of electrons present in the outermost shell is defined as valency. To measure valency, we can figure out the number of electrons that are required to complete the shell in which it is contained or losing excess electrons if present, once the filling is complete.
To find the valency of chlorine:
The atomic number of chlorine is 17
Number of electrons is equal to the number of protons in chlorine i.e., 17
The distribution of electrons in chlorine atom is K – 2, L – 8, M – 7
Hence, from the distribution of chlorine it is clearly evident that to fill the M shell only one electron is required. Therefore its valency is -1. i.e, one electron less
To find the valency of sulphur:
The atomic number of sulphur is 16
Number of electrons is equal to the number of protons in sulphur i.e., 16
The distribution of electrons in sulphur atom is K – 2, L – 8, M – 6
Hence, from the distribution of sulphur it is clearly evident that to fill the M shell two more electrons are required. Therefore its valency is -2, i.e., two electrons lesser.
To find the valency of magnesium:
The atomic number of magnesium is 12
Number of electrons is equal to the number of protons in magnesium i.e., 12
The distribution of electrons in magnesium atom is K – 2, L – 8, M – 2
Hence, from the distribution of magnesium it is clearly evident that to fill the M shell six more electrons are required. But M shell has two electrons only. It possesses lesser electrons than needed to fill the shell.
Thus, we say that the magnesium atom is not stable as the M shell has 2 electrons. Its valency is +2, meaning it has 2 electrons in excess.
Exercise-4.5 Page: 52
1. If the number of electrons in an atom is 8 and number of protons is also 8, then
(i) What is the atomic number of the atom? and
(ii) What is the charge on the atom?
Solution:
Given: Number of electrons = 8
Number of protons = 8
(i) The atomic number of an atom is the same as the number of protons in that atom, hence its atomic number is 8.
(ii) In an atom, the number of protons is equal to the number of electrons. Hence both the charges – positive and negative neutralize each other. Therefore, the atom does not possess any charge.
2. With the help of given Table, find out the mass number of oxygen and sulphur atom.
Table: Composition of Atoms of the First Eighteen Elements with Electron Distribution in Various Shells.
| Name of Element | Symbol | Atomic number | Number of Protons | Number of Neutrons | Number of electrons | Distribution of electrons
K L M N |
Valency | |||
| Hydrogen
Helium Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon Sodium Magnesium |
H
He Li Be B C N O F Ne Na Mg |
1
2 3 4 5 6 7 8 9 10 11 12 |
1
2 3 4 5 6 7 8 9 10 11 12 |
–
2 4 5 6 6 7 8 10 10 12 12 |
1
2 3 4 5 6 7 8 9 10 11 12 |
1
2 2 2 2 2 2 2 2 2 2 2 |
–
– 1 2 3 4 5 6 7 8 8 8 |
–
– – – – – – – – – 1 2 |
–
– – – – – – – – — – – |
1
0 1 2 3 4 3 2 1 0 1 2 |
| Aluminium
Silicon Phosphorus Sulphur Chlorine Argon |
Al
Si P S Cl Ar |
13
14 15 16 17 18 |
13
14 15 16 17 18 |
14
14 16 16 18 22 |
13
14 15 16 17 18 |
2
2 2 2 2 2 |
8
8 8 8 8 8 |
3
4 5 6 7 8 |
–
– – – – |
3
4 3,5 2 1 0 |
Solution:
(a) To find the mass number of Oxygen:
Number of protons = 8
Number of neutrons = 8
Atomic number = 8
Atomic mass number = Number of protons + number of neutrons = 8 + 8 = 16
Therefore, mass number of oxygen = 16
(b) To find the mass number of Sulphur:
Number of protons = 16
Number of neutrons = 16
Atomic number = 16
Atomic mass number = Number of protons + number of neutrons = 16 + 16 = 32
Exercise-4.6 Page: 53
1. For the symbol H, D and T, tabulate three subatomic particles found in each of them.
Solution:
The following table depicts the subatomic particles in Hydrogen (H), Deuterium (D), and Tritium(T).
| Isotope | Symbol | Mass no. | Atomic no. | No. of electrons | No. of protons | No. of neutrons |
| Hydrogen | H | 1 | 1 | 1 | 1 | 0 |
| Deuterium | D | 2 | 1 | 1 | 1 | 1 |
| Tritium | T | 3 | 1 | 1 | 1 | 2 |
2. Write the electronic configuration of any one pair of isotopes and isobar.
Solution:
(a) Isotopes: Isotopes are atoms which have the same number of protons but the number of neutrons differs. This leads to the variation in mass number too.
Example: Carbon molecule exists as 6C12 and 6C14 but when their electronic configuration is noticed, both have K-2; L-4
(b) Isobars: Isobars are atoms which have the same mass number but differ in the atomic number. Electronic configuration of an isobar pair is as follows,
Example: Electronic configuration of 20Ca40 – K-2; L-8; M-8; N- 2
Electronic configuration of 18Ar40 – K-2; L-8; M-8
Exercise Page: 54
1. Compare the properties of electrons, protons and neutrons.
Solution:
| Property | Electrons | Protons | Neutrons |
| Charge | Negatively charged | Positively charged | No charge. |
| Location | Located outside the nucleus | Located within the nucleus | Located inside the nucleus of an atom |
| Weight | Mass is negligible | 1 a.m.u | 1 a.m.u |
| Affinity | Attracted towards positively charged | Attracted towards negatively charged | Do not get attracted to any charged particle |
2. What are the limitations of J.J.Thomson’s model of the atom?
Solution:
The following are the limitations of the J.J. Thomson’s model of an atom.
- The model failed to explain the outcome of alpha particle scattering which was conducted by Rutherford. The model failed to depict why majority of these alpha particles pass through gold foil while some are diverted through small and big angles, while some others rebound completely, returning back on their path.
- It did not provide any experimental evidence and was established on imagination.
3. What are the limitations of Rutherford’s model of the atom?
Solution:
Following are the limitations of Rutherford’s model of the atom:
- There is no expected stability in the revolution of the electron in a circular orbit
- Charged particles radiate energy when accelerated thus causing the revolving electrons to lose energy and would fall into the nucleus
- Hence atoms must be highly unstable. Matter would not exist in their known form which clearly is an assumption as atoms are highly stable.
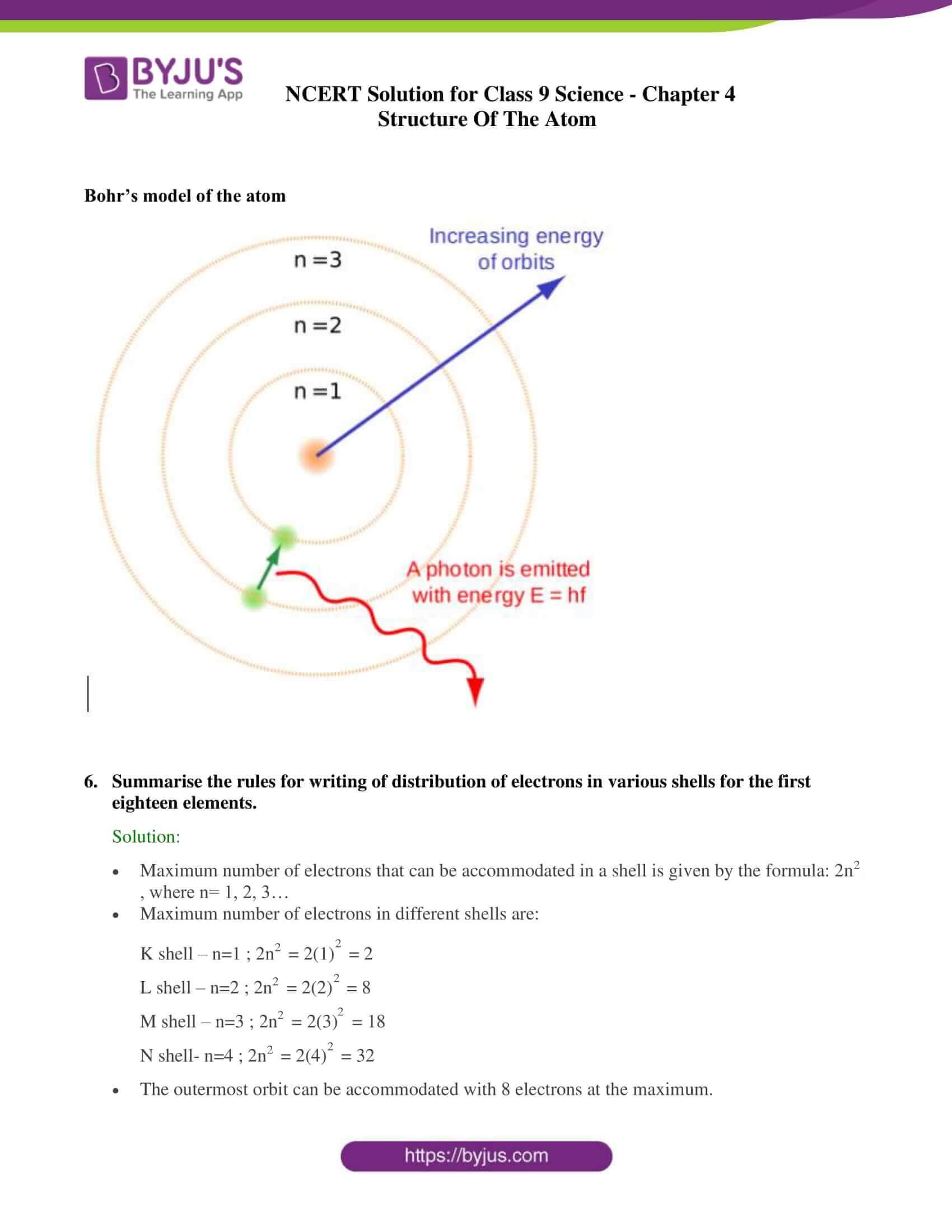
4. Describe Bohr’s model of the atom.
Solution:
- An atom holds the nucleus at the centre.
- Negatively charged electrons revolve around the nucleus.
- The atoms in it contains distinct orbits of electrons.
- Electrons do not radiate energy when they are in their orbits.
- The distinct orbits are named as K, L, M, N orbits. Numbers used to denote them are n=1, 2, 3, 4

5. Compare all the proposed models of an atom given in this chapter.
Solution:
| Thomson | Rutherford | Bohr |
| ● Sphere is positively charged
●Electrons are negatively charged and scattered all through the inside of the sphere. ● Positively charged = negatively charged ● The net charge in the atom is zero. |
● The nucleus is at the centre and is positively charged holding the entire mass.
● Electrons are negatively charged revolving in a well-defined path ●In comparison with the nucleus, the size of the atom is very large. |
●Nucleus is present at the centre and is positively charged
● Electrons are negatively charged, revolving around but do not radiate energy. ● The distinct orbits are labelled as K, L, M, N |
6. Thomson’s Model of Atom

7. Rutherford’s Model of Atoms

8. Bohr’s model of the atom

Summarise the rules for writing of distribution of electrons in various shells for the first eighteen elements.
Solution:
- Maximum number of electrons that can be accommodated in a shell is given by the formula: 2n2, where n= 1, 2, 3…
- Maximum number of electrons in different shells are:
K shell – n=1 ; 2n2 = 2(1)2 = 2
L shell – n=2 ; 2n2 = 2(2)2 = 8
M shell – n=3 ; 2n2 = 2(3)2 = 18
N shell- n=4 ; 2n2 = 2(4)2 = 32
- The outermost orbit can be accommodated with 8 electrons at the maximum.
- The electrons are not taken in unless the inner shells are filled which are filled step-wise, hence the highest element has K-2; L-8 ; M-8 distribution of electrons.
9. Define valency by taking examples of silicon and oxygen.
Solution:
The definite combining capacity of the atoms of each element, wherein electrons are lost, gained or shared to make the octet of electrons present in the outermost shell is defined as valency. To measure valency, we can figure out the number of electrons that are required to complete the shell in which it is contained or losing excess electrons if present, once the filling is complete.
Example : To find the valency of silicon:
The atomic number of silicon is 14
Number of electrons is equal to the number of protons in silicon i.e., 14
The distribution of electrons in silicon atom is K – 2, L – 8, M – 4
Hence, from the distribution of silicon it is clearly evident that to fill the M shell 4 electrons are required. Therefore its valency is 8-4=4.
To find the valency of oxygen:
The atomic number of oxygen is 8
Number of electrons is equal to the number of protons in oxygen i.e., 8
The distribution of electrons in oxygen atom is K – 2, L – 6
Hence, from the distribution of oxygen it is clearly evident that to fill the M shell 6 more electrons are required. Therefore its valency is 8-6=2.
10. Explain with examples
(i) Atomic number,
(ii) Mass number,
(iii) Isotopes and
(iv) Isobars.
Give any two uses of isotopes.
Solution:
(i) The number of positively charged protons present in the nucleus of an atom is defined as the atomic number and is denoted by Z. Example: Hydrogen has one proton in its nucleus, hence its atomic number is one.
(ii) The total number of protons and neutrons present in the nucleus of an atom is known as the mass number. It is denoted by A. 20Ca40 . Mass number is 40. Atomic number is 20.
(iii) The atoms which have the same number of protons but different number of neutrons are referred to as isotopes. Hence the mass number varies.
Example: The most simple example is the Carbon molecule which exists as 6C12 and 6C14
(iv) Isobars: Isobars are atoms which have the same mass number but differ in the atomic number.
Examples are, 20Ca40and 18Ar40
Uses of isotopes:
- The isotope of Iodine atom is used to treat goitre and iodine deficient disease.
- In the treatment of cancer, an isotope of cobalt is used.
- Fuel for nuclear reactors is derived from the isotopes of the Uranium atom.
11. Na+ has completely filled K and L shells. Explain.
Solution:
The atomic number of sodium is 11. It has 11 electrons in its orbitals wherein the number of protons is equal to the number of electrons. Hence, its electronic configuration is K-2 ; L-8 ; M-1 ; The one electron in the M shell is lost and it obtains a positive charge since it has one more proton than electrons, and obtains a positive charge, Na+ . The new electronic configuration is K-1 ; L-8 which is the filled state. Hence it is very difficult to eliminate the electron from a filled state as it is very stable.
12. If bromine atom is available in the form of, say, two isotopes 35Br79 (49.7%) and 35Br81 (50.3%), calculate the average atomic mass of Bromine atom.
Solution:
The atomic masses of two isotopic atoms are 79 (49.7%) and 81 (50.3%).
Thus, total mass = (79 * 49.7 / 100) + (81 * 50.3 / 100) = 39.263 + 40.743 = 80.006 u
13. The average atomic mass of a sample of an element X is 16.2 u. What are the percentages of isotopes 8X16 and 8X18 in the sample?
Solution:
Let the percentage of 8X16 be ‘a’ and that of 8X18 be ‘100-a’.
As per given data,
16.2u = 16 a / 100 + 18 (100-a) /100
1620 = 16a + 1800 – 18a
1620 = 1800 – 2a
a = 90%
Hence, the percentage of isotope in the sample 8X16 is 90% and that of
8X18 = 100-a = 100- 90=10%
14. If Z=3, what would be the valency of the element? Also, name the element.
Solution:
Given: Atomic number, Z = 3
The electronic configuration of the element = K-2; L-1, hence its valency = 1
The element with atomic number 3 is Lithium.
15. Composition of the nuclei of two atomic species X and Y are given as under
X Y
Protons = 6 6
Neutrons = 6 8
Give the mass numbers of X and Y. What is the relation between the two species?
Solution:
Mass number of X: Protons + neutrons = 6+6 = 12
Mass number of Y: Protons + neutrons = 6+8 = 14
They are the same element as their atomic numbers are the same.
They are isotopes as they differ in the number of neutrons and hence their mass numbers.
16. For the following statements, write T for true and F for false.
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
(b) A neutron is formed by an electron and a proton combining together. Therefore it is neutral.
(c) The mass of an electron is about 1/2000 times that of proton.
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine.
Solution:
(a) Statement is False
(b) Statement is False
(c) Statement is True
(d) Statement is False
17. Put a tick(✓) against correct choice and cross(x) against wrong choice in questions 15, 16 and 17.
Rutherford’s alpha – particle scattering experiment was responsible for the discovery of
(a) Atomic nucleus
(b) Electron
(c) Proton
(d) Neutron
Solution:
(a) Atomic nucleus
Isotopes of an element have
(a) The same physical properties
(b) Different chemical properties
(c) Different number of neutrons
(d) Different atomic numbers.
Solution:
(c) Different number of neutrons
18. Number of valence electrons in Cl– ion are:
(a) 16
(b) 8
(c) 17
(d) 18
Solution:
(b) 8
Electronic distribution of Cl is K-2, L-8, M-7. Valence electrons are 7, hence chlorine gains one electron for the formation of Cl–. Therefore, its valency is 8.
19. Which one of the following is a correct electronic configuration of Sodium?
(a) 2, 8
(b) 8, 2, 1
(c) 2, 1, 8
(d) 2, 8, 1
Solution:
(d) 2, 8, 1
Complete the following table.
| Atomic number | Mass number | Number of neutrons | Number of Protons | Number of electrons | Name of the atomic species |
| 9
16 – – – |
–
32 24 2 1 |
10
– – – 0 |
–
– 12 1 1 |
–
– – – 0 |
–
Sulphur – – – |
Solution:
The following table depicts the missing data:
Atomic number(Z) =Number of protons
Mass number = Number of neutrons + atomic number
(or)
Mass number(A) = Number of neutrons + number of neutrons
| Atomic number | Mass number | Number of neutrons | Number of Protons | Number of electrons | Name of the atomic species |
| 9
16 12 1 1 |
19
32 24 2 1 |
10
16 12 1 0 |
9
16 12 1 1 |
9
16 12 1 0 |
Fluorine
Sulphur Magnesium Deuterium Hydrogen |
| Also Access |
| NCERT Exemplar Solutions for Class 9 Science Chapter 4 |
| CBSE Notes for Class 9 Science Chapter 4 |
NCERT Solutions for Class 9 Science Chapter 4 – Structure Of The Atom
Chapter 4 Structure of the atom of NCERT Class 9 Science is categorized under Unit I – Matter – Its nature and behaviour which fundamentally deals with the physical nature of matter and what constitutes the particles that make up matter. The whole unit of Matter makes up for 23 marks out of which the Structure of the atom chapter is allocated 10 marks. In order to score maximum marks, students should practise using the Science NCERT Solutions for Class 9.
This chapter has a good weightage. Thorough knowledge of all the concepts covered in this chapter will help you achieve up to 10 marks from this chapter alone. Around 2 questions with 5 marks each appear in the CBSE Term II examination from this chapter and are expected to appear in the following year too.
List of subtopics covered in Class 9 Science Chapter 4 Structure Of The Atom:
| Section | Topic/Subtopic |
| 4.1 | Charged particles in matter |
| 4.2 | The structure of an atom |
| 4.2.1 | Thomson’s model of an atom |
| 4.2.2 | Rutherford’s model of an atom |
| 4.2.3 | Bohr’s model of an atom |
| 4.2.4 | Neutrons |
| 4.3 | How are electrons distributed in different orbits(shells)? |
| 4.4 | Valency |
| 4.5 | Atomic number and mass number |
| 4.5.1 | Atomic number |
| 4.5.2 | Mass number |
| 4.6 | Isotopes |
| 4.6.1 | Isobars |
Students can best utilise the NCERT Solutions for Class 9 Science Chapter 4 to make any quick references to comprehend the above and other complex topics with ease. Further, these solutions can be viewed both online and offline as a downloadable PDF.
List of Exercises in Class 9 Science Chapter 4
Number 4.1 – Charged particles in matter 2 Question ( 2 short)
Number 4.2 – The structure of an atom 4 Question ( 4 short)
Number 4.2.4 – Neutrons 2 Question ( 2 short)
Number 4.3 – How are electrons distributed
indifferent orbits(shells)? 2 Question ( 1 long, 1 short)
Number 4.4 – Valency 1 Question ( 1 long)
Number 4.5 – Atomic number and mass number 2 Question ( 2 long)
Number 4.6 – Isotopes 2 Question ( 1 long, 1 short)
Exercise Solutions – 19 Question ( 6 long, 9 short, 4 MCQ)
NCERT Solutions for Class 9 Science Chapter 4 – Structure of the Atom
In this chapter, we get enlightened about the discovery of electrons and protons that is credited to J.J. Thomson and E.Goldstein, respectively. As per the theory by J.J.Thomson, electrons are implanted in the positive sphere. The discovery of the atomic nucleus was forwarded by Rutherford’s alpha-particle scattering experiment. We also learn about how Neil Bohr’s model was successful as he explained how electrons are distributed in different orbits around the nucleus.
This chapter also briefs about the discovery of neutrons in an atom proposed by J.Chadwick, hence the conclusion about the existence of three sub-atomic particles i.e., protons, neutrons, and electrons. It also sheds light upon concepts such as valency, atomic number and mass number, which are essential to identify elements and their behavioural properties. Learn more about these concepts by accessing the NCERT Solutions for Class 9 of this chapter of Science.
Overall, it helps us answer the basic concepts of what sets an atom apart from atoms of other elements, the indivisibility concept of atoms, representation of elements through mass number and atomic mass, as well as the basic constituents of an atom.
Key Features of NCERT Solutions for Class 9 Science Chapter 4 – Structure of The Atom
- NCERT Solutions enable students to prepare for CBSE Class 9 Term II examination stress-free.
- Calculations and electronic configurations have been explained in detail while solving.
- Diagrams have been used wherever necessary to be able to learn visually.
- Numericals involving finding valency of elements have been worked out in detail.
- Comparison questions involve the use of tables to help learn and understand better.
- Pointers have been used in the solutions to remember at a glance.
Frequently Asked Questions on NCERT Solutions for Class 9 Science Chapter 4
List the topics and subtopics covered in Chapter 4 of NCERT Solutions for Class 9 Science.
4.1 Charged particles in matter
4.2 The structure of an atom
4.2.1 Thomson’s model of an atom
4.2.2 Rutherford’s model of an atom
4.2.3 Bohr’s model of an atom
4.2.4 Neutrons
4.3 How are electrons distributed in different orbits(shells)?
4.4 Valency
4.5 Atomic number and mass number
4.5.1 Atomic number
4.5.2 Mass number
4.6 Isotopes
4.6.1 Isobars
How many exercises are present in Chapter 4 of NCERT Solutions for Class 9 Science?
Number 4.1 – Charged particles in matter 2 Questions ( 2 short)
Number 4.2 – The structure of an atom 4 Questions ( 4 short)
Number 4.2.4 – Neutrons 2 Questions ( 2 short)
Number 4.3 – How are electrons distributed
Indifferent orbits(shells) – 2 Questions ( 1 long, 1 short)
Number 4.4 – Valency 1 Question ( 1 long)
Number 4.5 – Atomic number and mass number 2 Questions ( 2 long)
Number 4.6 – Isotopes 2 Questions ( 1 long, 1 short)
Exercise Solutions – 19 Questions ( 6 long, 9 short, 4 MCQ)
Where can I find the NCERT Solutions for Class 9 Science Chapter 4 online?